Senior managers in the drug industry are responsible for the effectiveness of this system which is known as the pharmaceutical quality system pqs.
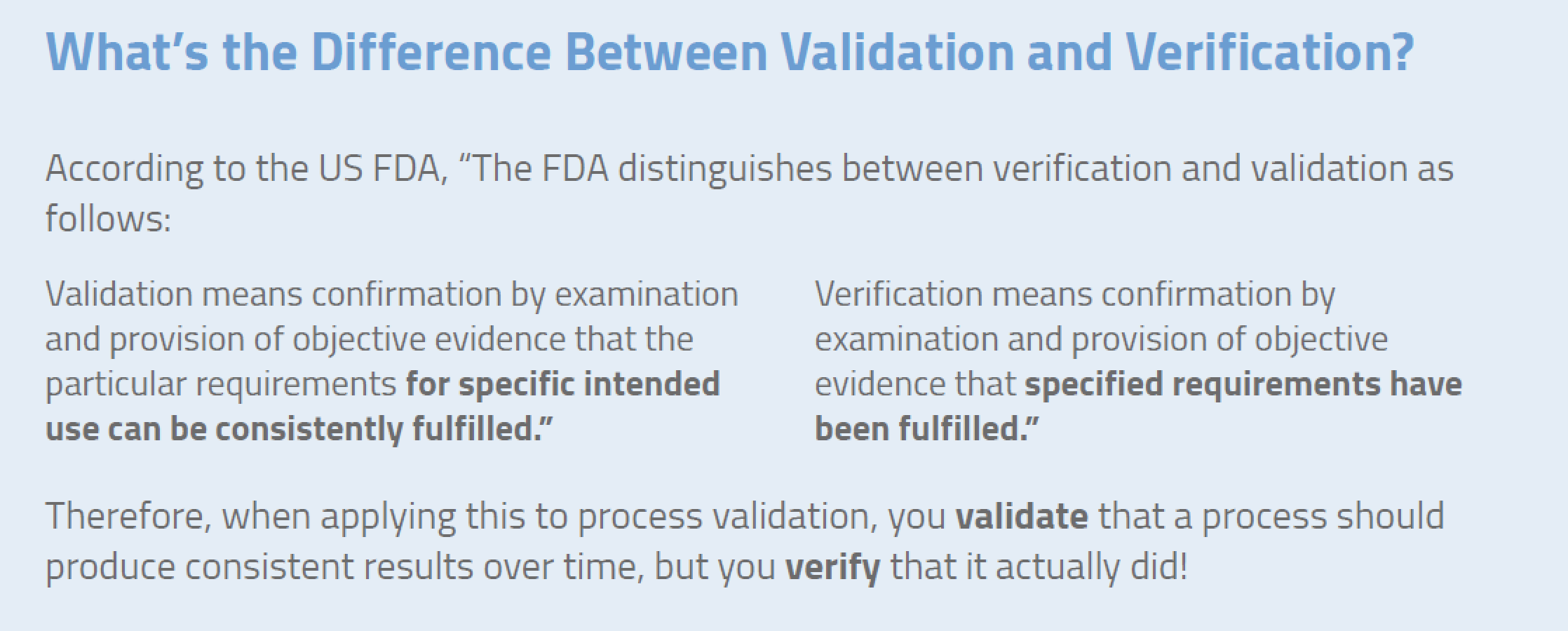
Fda quality management system definition.
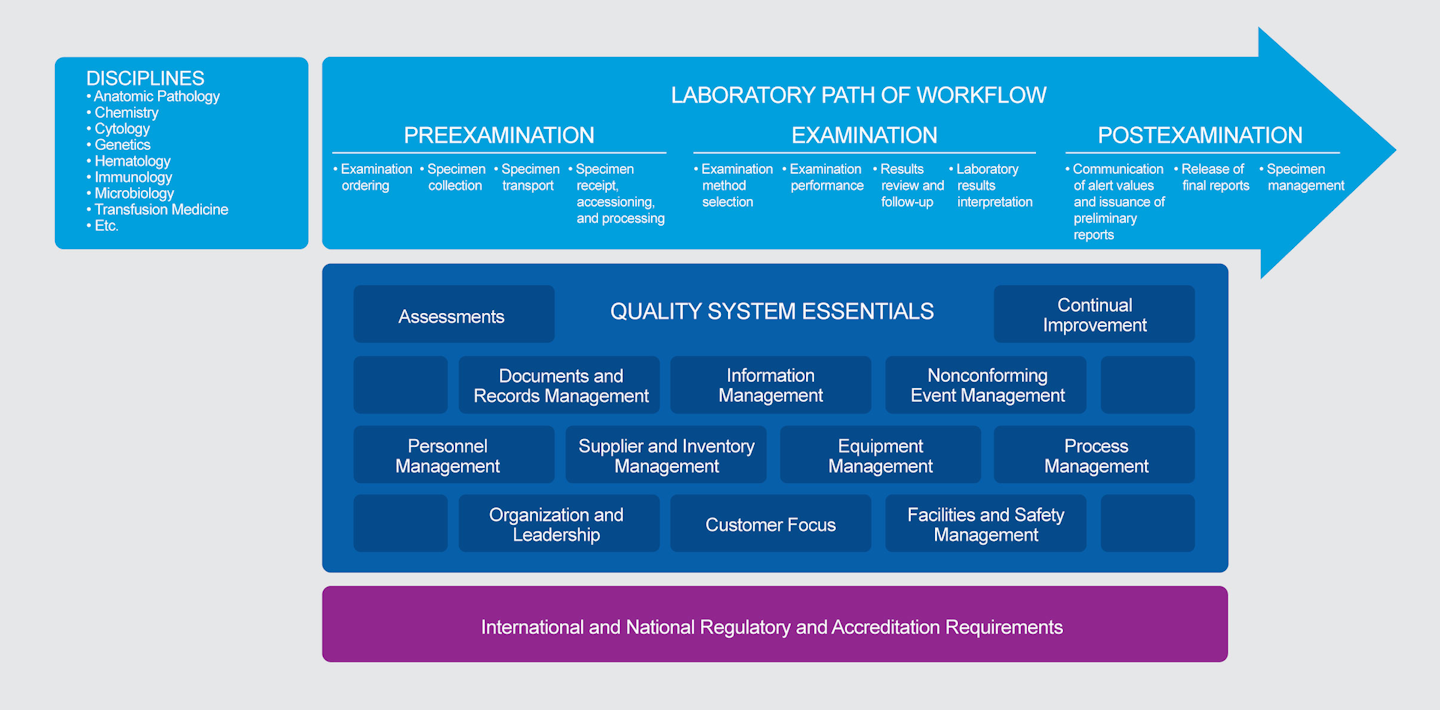
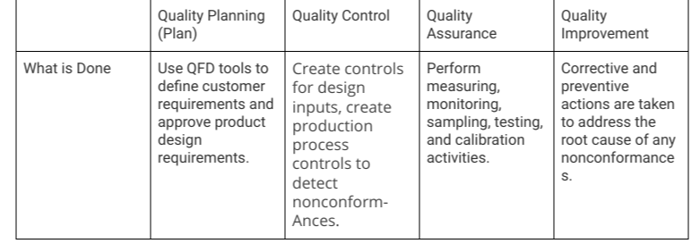
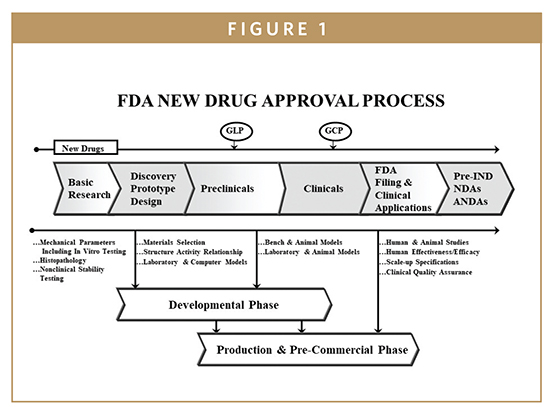
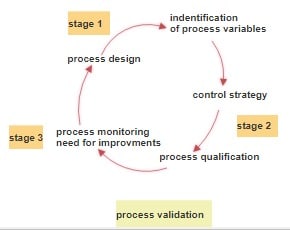
Improvement and risk management in the drug manufacturing process.
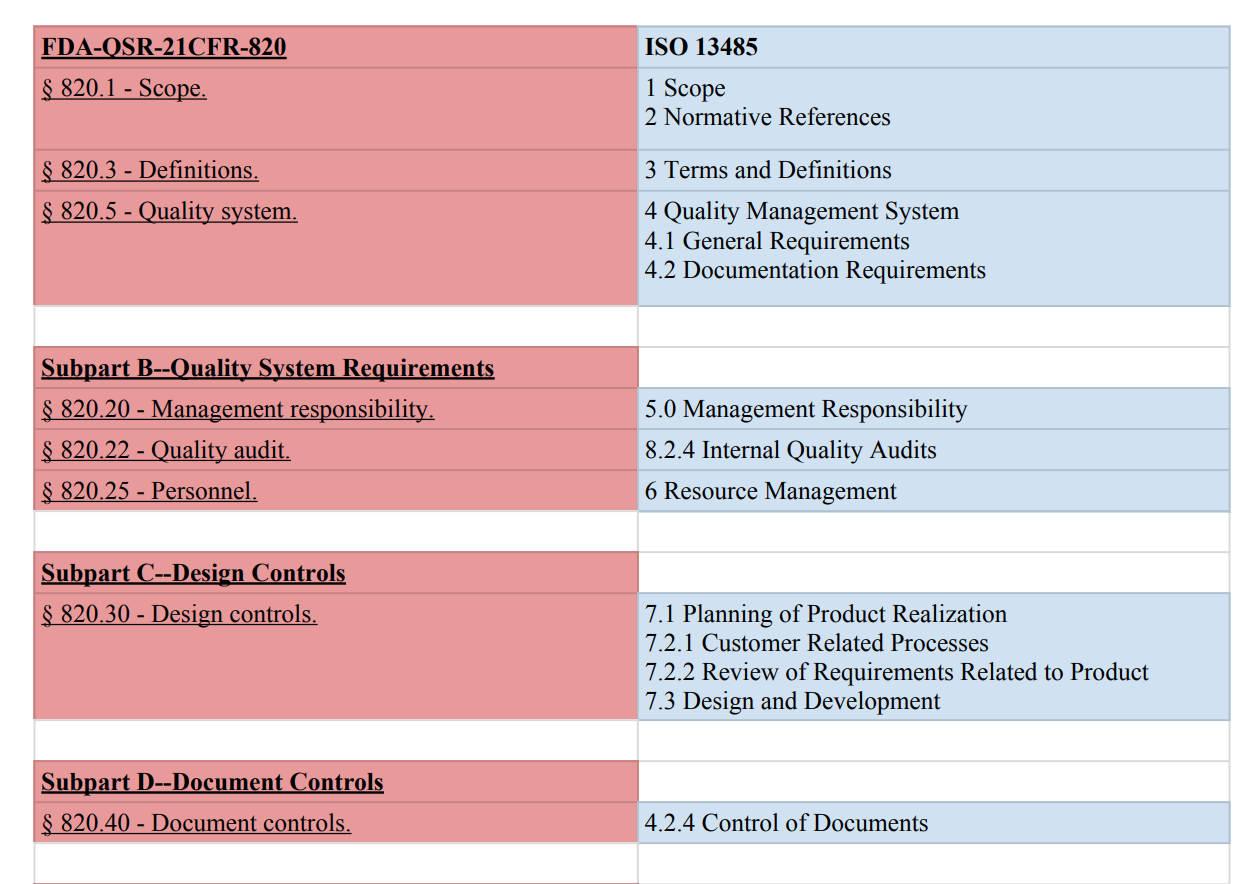
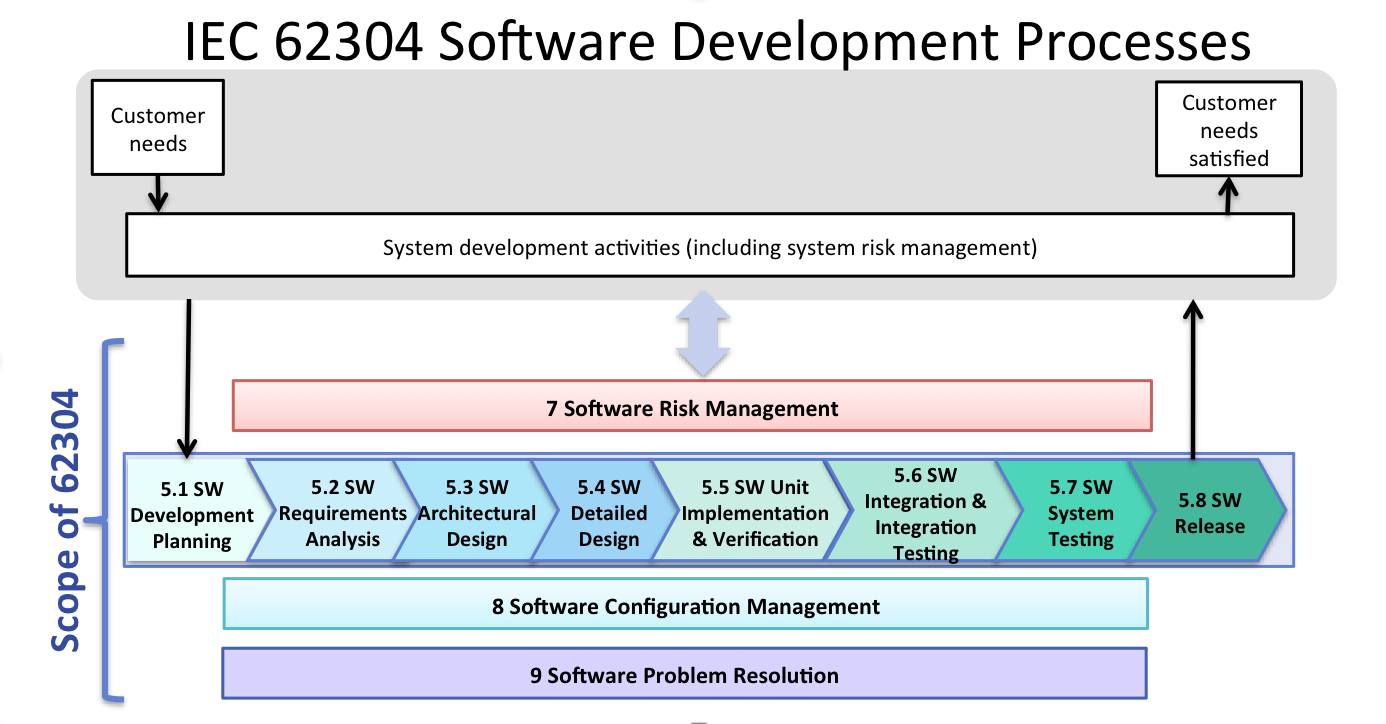
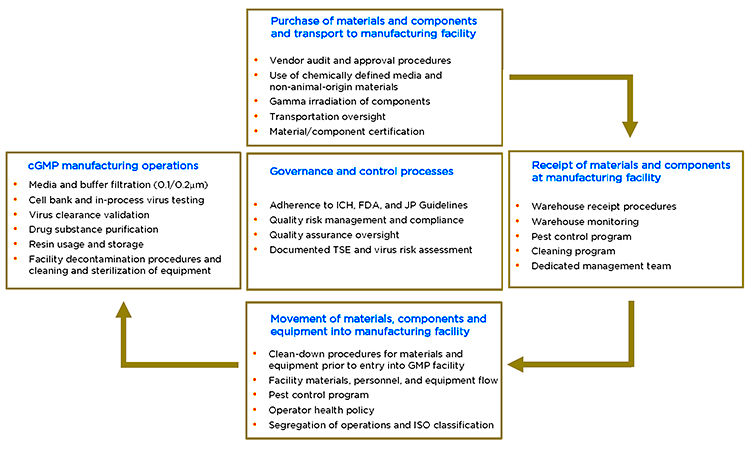
In the guidance referenced above fda points to cgmp regulations which broadly assign the quality unit to create monitor and implement a quality system regulators also define its scope and impact on other units noting that while the quality unit should not take on the responsibilities of other units of a manufacturer s organization such as the responsibilities handled by manufacturing personnel engineers and development scientists it does not absolve other units of their.
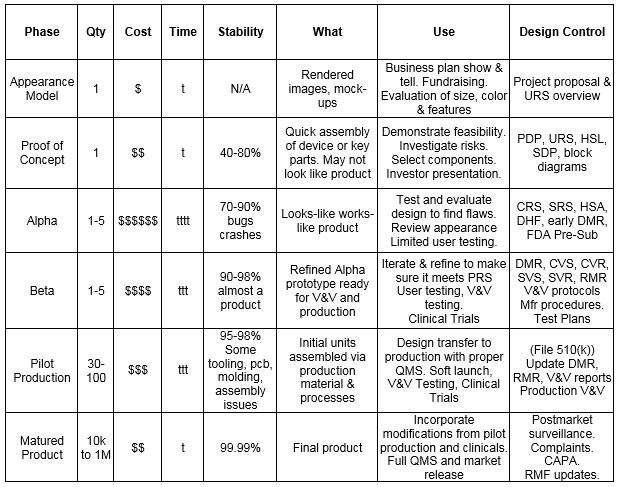
A quality system adopted by a manufacturer can be tailored to fit the specific environment taking into account factors such.
Means the organizational structure responsibilities procedures processes and resources for implementing quality management.
Management with executive responsibility shall review the suitability and effectiveness of the quality system at defined intervals and with sufficient frequency according to established procedures to ensure that the quality system satisfies the requirements of this part and the manufacturer s established quality policy and objectives.
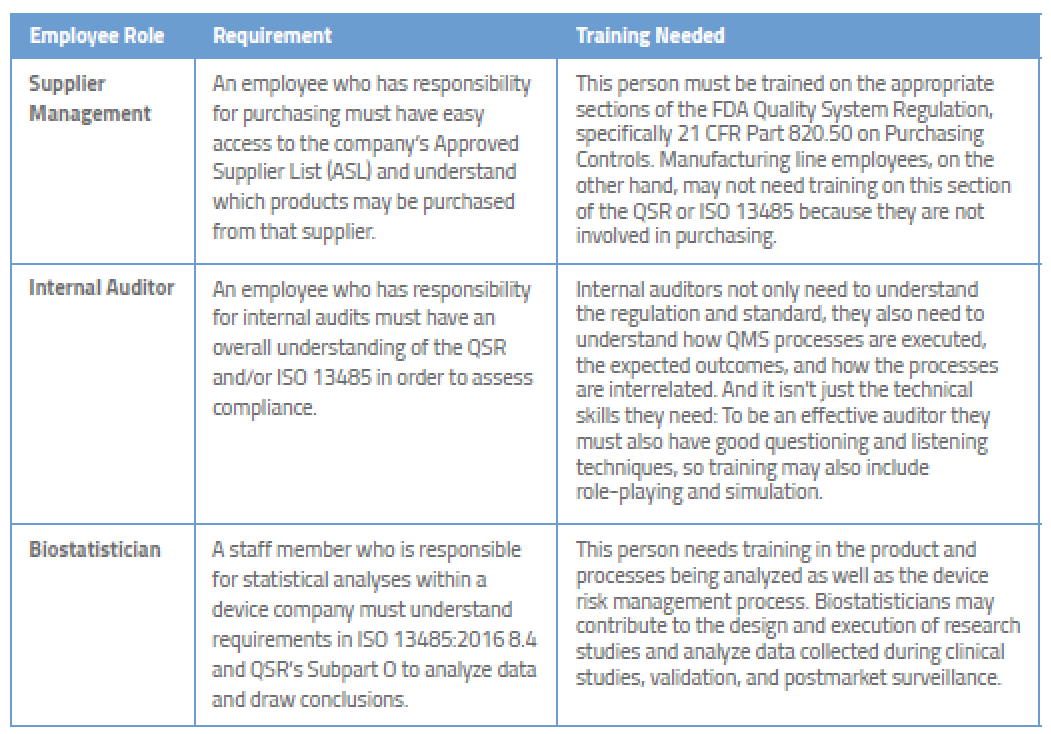
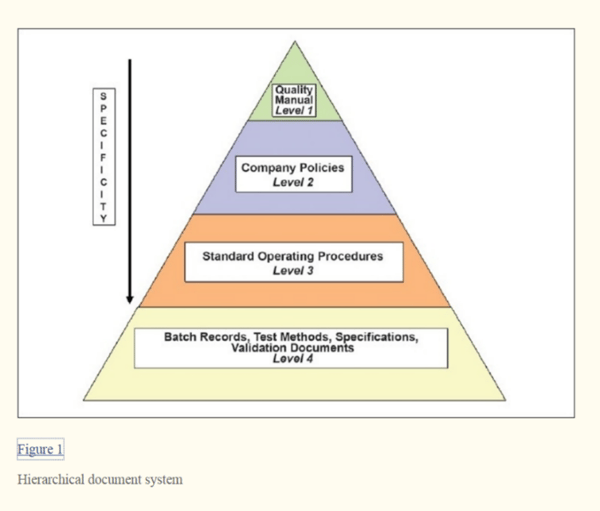
Quality production laboratory materials facilities and equipment packaging and labeling management responsibility.
The definition of quality policy is provided in the quality system regulation.
Quality assurance programme means a defined system including personnel which is independent of study conduct and is designed to assure test facility management of compliance with these.
It means the overall intentions and directions of an organization with respect to quality.