Form and content of the unique device identifier udi draft guidance for industry and food and drug administration staff 08 14 2016 gudid submission.
Fda unique device identification udi rule.
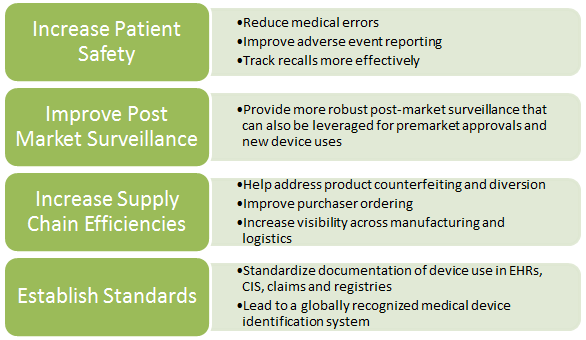
Unique device identification system.
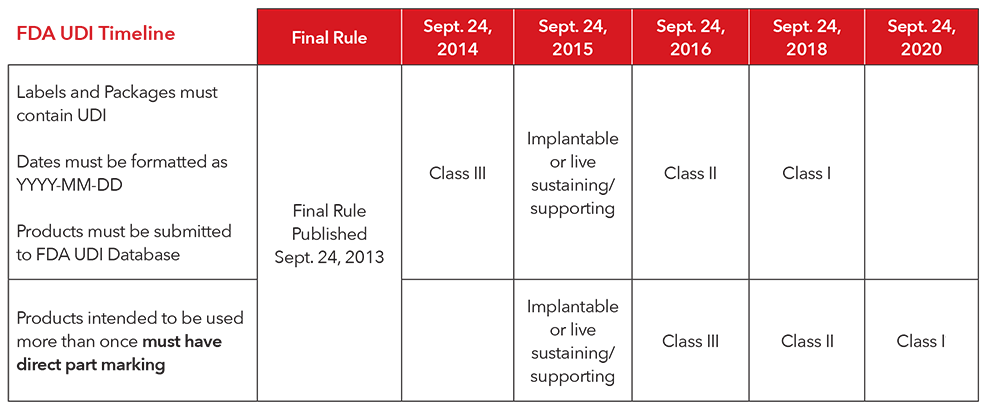
Include a unique device identifier udi issued under an fda accredited issuing agency s udi system on device labels device packages and in some instances directly on the device.
A class ii device that is required to be labeled with a udi must bear a udi as a permanent marking on the device itself if the device is a device.
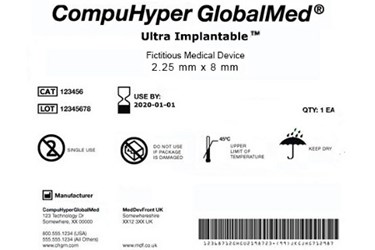
This rule requires the label of medical devices to include a unique device identifier udi except where the rule provides for an exception or alternative placement.
Food and drug administration fda created unique device identification often abbreviated udi a rule that requires medical device manufacturers to update their products with a unique device identifier that includes both device and production identifiers such as expiration date and lot or serial number.
The labeler must submit product information concerning devices to fda s global unique device identification database gudid unless subject to an exception or alternative.
The unique device identification system final rule udi rule requires device labelers typically the manufacturer to.
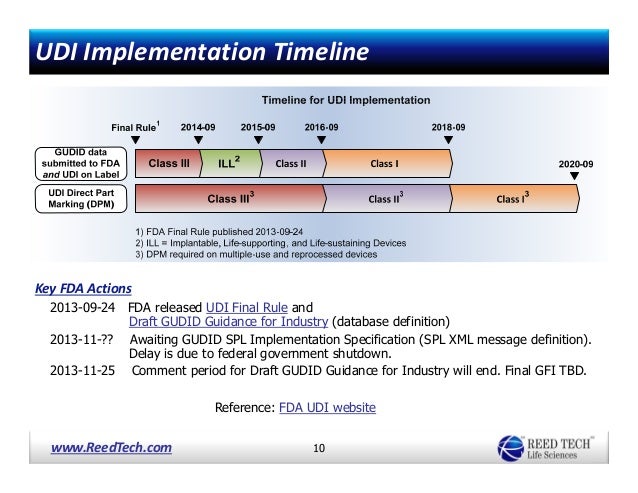
On september 24 2013 fda published a final rule establishing a unique device identification system the udi rule.
Unique device identification udi the u s.
The system established by this rule would require the label of medical devices and device packages to include a unique device identifier udi except where the rule provides for alternative.
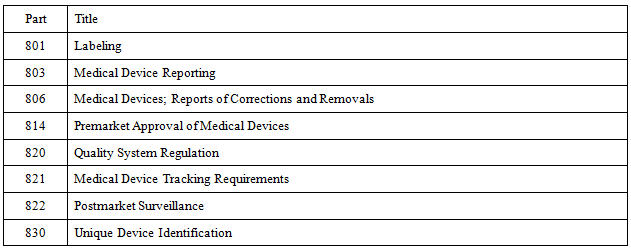
Erg under contract to fda and are presented in the full report unique device identification udi for medical devices.